Concept of Hybridisation and its Applications
Concept of Hybridisation and its Applications: Overview
This topic covers concepts such as sp Hybridisation, sp2 Hybridisation, sp3 Hybridisation, Hybridisation, Rules for Hybridisation, sp3d Hybridisation, sp3d2 and d2sp3 Hybridization, dsp2 Hybridisation, Hybrid Orbitals, etc.
Important Questions on Concept of Hybridisation and its Applications
Which of the following represent the given mode of hybridisation from left to right?
In which of the following species, the underlined carbon atom is hybridised?
Which is the following has the regular tetrahedral structure?
The state of hybridisation of boron and oxygen atoms in boric acid molecule are respectively –
Write the definition of hybridisation.
The hybridisation in molecule is
How many orbitals take part in hybridisation.
What is hybridization? Give an example.
Which of the following molecules do not have regular geometry?
molecule is formed by hybrid orbitals?
Among the following transformations, the hybridisation of the central atom remains unchanged in
The hybridisations of and shown in the following compound
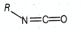
respectively, are
The hybridisation of xenon atom in is
Hybridisation of both 'c' in acetylene molecule
In acetylene molecule, there are
The bond angles formed by different hybrid orbitals are in the order:
Which of the following is the correct order regarding the electronegativity of hybrid orbitals of carbon?
In an octahedral structure, the pair of d-orbitals involved in hybridisation is
Assertion. molecule is planar but is pyramidal.
Reason. atom is smaller than
Assertion. does not have a tetrahedral structure.
Reason. in has two lone pairs
